One of the most common and financially impactful diseases in modern dairy production is mastitis. This inflammation of the mammary gland is mainly caused by bacteria which are either Gram-positive or Gram-negative.
Clinical and subclinical mastitis
Mastitis cases can be clinical or subclinical. Clinical mastitis is an inflammatory response to infection causing visibly abnormal milk (e.g., off-colour or containing fibrin clots). As the extent of the inflammation increases, changes in the udder (such as swelling, heat, pain, redness) may also be apparent. Clinical cases vary from mild or moderate to severe.
Subclinical mastitis limits milk production and represents an important barrier to profitable dairy production. The symptoms are not visible to the naked eye.
The somatic cell count (SCC) is most often used as an indicator of milk quality as well as the presence of otherwise hidden subclinical mastitis. It is defined as the number of cells per ml of milk. The function of somatic cells is to fight possible infections and to facilitate the repair of damaged tissue. Leukocytes represent the majority of the SCC, up to 90% according to some sources, and the rest are epithelial cells. Leukocyte numbers rise significantly in an immune response to different mastitis pathogens. An SCC number of up to 100,000 indicates uninfected milk. A threshold of 200,000 very likely indicates an infection. SCC results of more than 200,000 indicate a high probability of at least one quarter of the udder being infected with a pathogen. An SCC number exceeding 300,000 indicates the presence of a significant pathogen. The SCC threshold of milk not being fit for human consumption in the EU is 400,000.
Causative bacteria
Mastitis can be caused by Gram-negative bacteria. E. coli, which is a Gram-negative bacteria, is responsible for 80% of the cases of clinical mastitis (Bradley et al., 2007) and this is called coliform mastitis. Clinical and subclinical mastitis can also be caused by Gram-positive bacteria (Streptococcus spp., Staphylococcus spp.) and can vary in severity.
Clinical mastitis with mild or moderate clinical signs can be treated with a non-antimicrobial approach including nonsteroidal anti-inflammatory (NSAID) products, frequent milking, and fluid therapy (Suojala et al., 2013). For more severe cases of mastitis, antibiotic intramammary syringes are required for treatment. In the past, fluoroquinolones and 3rd or 4th generation cephalosporins were routinely used to treat clinical mastitis. However, both are critically important drugs, and should not be used in veterinary medicine without due cause.
Apart from the intramammary application route (Albiotic®, Huvepharma®), parenteral (application by intravenous, intramuscular, or subcutaneous injection) treatment during lactation should not be underestimated, especially in severe cases of mastitis. There are very few active molecules which have been authorised for parenteral treatment of mastitis. One such molecule is tylosin (Pharmasin® 200), a member of the macrolide group.
Pharmacokinetics of Pharmasin® 200 Solution for Injection (tylosin base)
Tylosin base is a weak organic base in its pure form. It is lipophilic, which means that it dissolves in lipids or fats. Milk has a high fat content and a pH of around 6.7 (even in cases of mastitis). Tylosin easily crosses the blood tissue barrier when an acidic environment is present (often the case with infections) and moves into the lipophilic environment where it becomes ion-trapped within macrophage cells. Macrophages, the first line of defence during infection, are slightly acidic, thus attracting tylosin. Upon entering macrophages, tylosin receives and H+ ion, becomes ionised and consequently trapped. This is one of the reasons why concentrations of tylosin in the milk in cases of mastitis can be up to 20 times their plasma levels (Gingerich et al., 1977).
The efficacy of tylosin in vivo was evaluated in a study by McDougall et al. (2007)
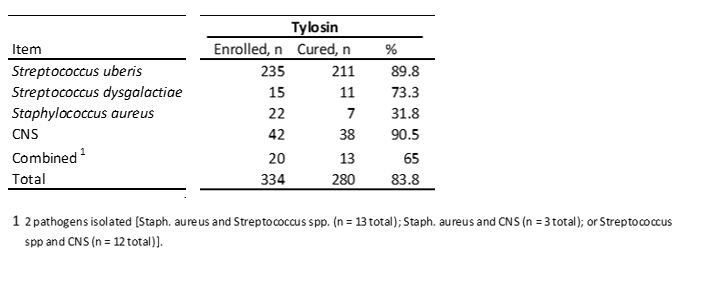
McDougall's study concluded a cure rate of 83.8% for clinical mastitis caused by organisms sensitive to tylosin.
According to the study, if infections were present and treated in early lactation, bacteriological cure rates were higher compared with infections present later in lactation. This was contrary to studies showing similar cure rates regardless of the time postpartum at which infections were treated (Sol et al., 2000).
Pharmacodynamics of Pharmasin® 200 Solution for Injection (tylosin base)
Tylosin is a time-dependent antibiotic. A time-dependent antibiotic requires the maintenance of plasma concentrations higher than the MIC90 (minimum inhibitory concentration) for at least 40% of the time between consecutive doses (Martinez et al., 2013).
The MIC90 of Staphylococcus aureus and coagulase-negative Staphylococcus spp. requires a plasma concentration of at least 2 μg/ml (Thomas et al., 2015).
The administration of tylosin base intravenously achieved concentrations above the MIC90 for 41% of the dosage interval. Intramuscular application of 10 mg/kg body weight of tylosin base, once per day, provided T > MIC90 for 43% of the dosage interval. It is thus clear that both intravenous and intramuscular administration of Pharmasin® 200 Solution for Injection achieve plasma levels in excess of the MIC90 for these mastitis-causing bacteria.
Cmax is the maximum (or peak) serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administered and before the administration of a second dose. The concentration of tylosin in both plasma and in milk are important when considering the treatment of mastitis.
The study by Thomas et al. (2015) compared the routes of administration of a tylosin base: intravenous and intramuscular, in both milk and plasma. The study demonstrated that the intravenous route of administration is preferred when starting treatment of severe clinical mastitis. This is due to significantly higher Cmax values after intravenous administration in comparison to the intramuscular route. In addition, with intravenous administration, there were high milk concentrations (2.49 ± 2.61 μg/ml) within the first thirty minutes. While an initial intravenous injection is preferred at the start of treatment, the study showed that subsequent intramuscular injections of tylosin base will maintain concentrations within the udder on following treatment days (Figures 1 and 2).
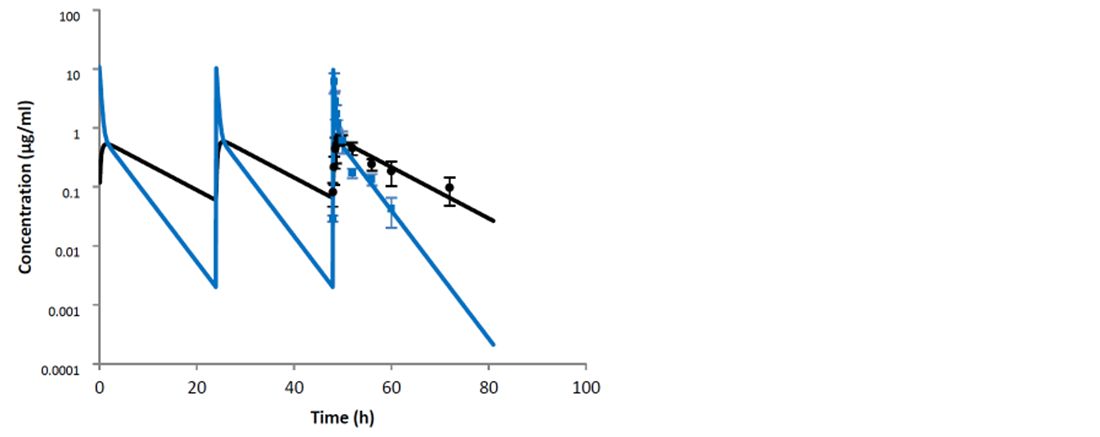
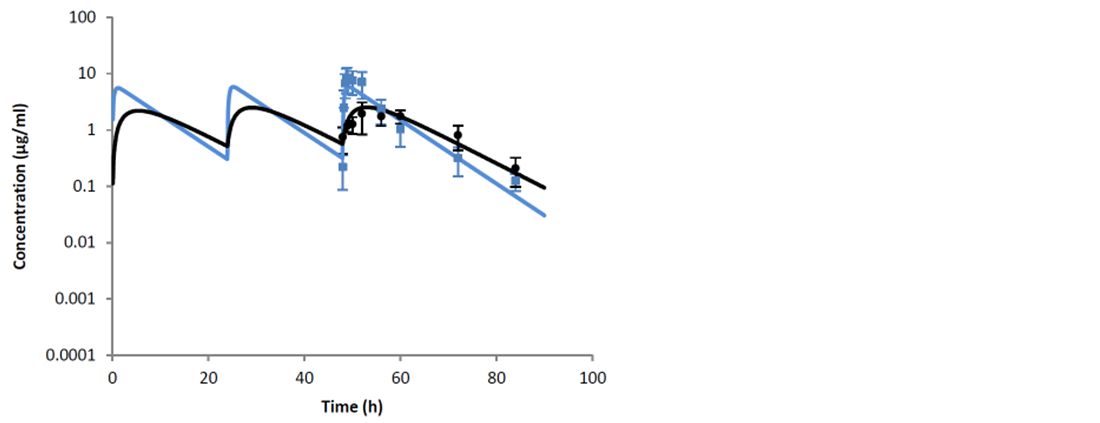
The authors concluded that the obtained data allow a recommendation of an initial intravenous injection of tylosin base, followed by two doses (12 hours apart) by intramuscular treatment.
Depondt et al. (2017) looked at the advantages of tylosin base in the treatment of severe clinical mastitis. This study confirmed the reported results for the deposition of high concentrations of tylosin in milk compared to plasma concentrations after intramuscular injection (Avci and Elmas, 2014).
Conclusion
Pharmasin® 200 Solution for Injection expresses some of the most desirable pharmacodynamic and pharmacokinetic characteristics for the parenteral treatment of mastitis including:
- A weak base
- An affinity towards the target tissue (udder)
- Liposolubility
- High tissue penetration
- Easy passage through the blood milk barrier
- Tissue trapping effect
These characteristics make tylosin more likely than less lipophilic compounds to achieve concentrations in the udder greater than the MIC90 (Ziv, 1980).
This is why Pharmasin® 200 Solution for Injection is an ideal product for parenteral treatment of both clinical and subclinical mastitis.
References are available on request


